Covaxin Who Approval Status Today
The WHO on October 27 sought more clarifications from Bharat Biotech manufacturer of COVAXIN in order to approve the made-in-India COVID-19 vaccine in its Emergency Use Listing. The FDA has cited insufficient information on Covaxin to grant approval to its emergency use authorisation request for this vaccine.
Updated Aug 12 2021 953 AM IST.

Covaxin who approval status today
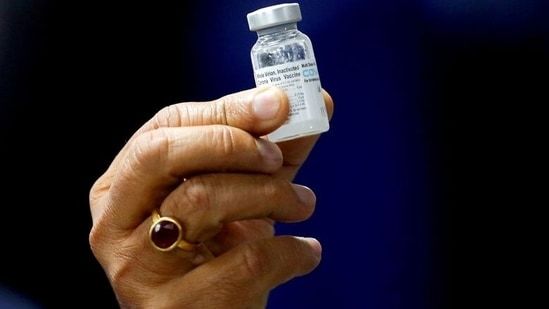
. The WHO will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week the global health body said on Tuesday. Covaxin decision date to be confirmed says WHO Premium A health worker preparing a dose from a vial of Covaxin a Covid-19 vaccine. He also stressed that in a few days India will achieve administration of 1. She had concurred that Covaxins safety profile so far met the WHO benchmarks.The Expression of Interest EOI for the emergency use approval of Covaxin was submitted by the company on April 19 2021 after which WHO demanded more data from the company. As Bharat Biotech had submitted the data required for WHOs approval on July 9 2021 the process is. Bharat Biotechs Covaxin is likely to get global authorisation from the World Health Organisation WHO by the end of this month. WHO decision on emergency use listing for Covaxin likely next week The countrys indigenous Covid-19 vaccine has not yet received emergency use approval from WHO.
WHO seeks additional clarification from Bharat Biotech decision likely on November 3 The technical advisory group will now meet on November 3 for a. Top health experts are of the view that Covaxins approval shouldnt be. WHO to take final call on approval of Covaxin next week. Documents detailing the WHO expert committees agenda for the meeting show that it will discuss the assessment status of pending applications for vaccine candidates including Covaxin.
Heres a list of countries that have approved the Bharat Biotech vaccine As the vaccine is yet to receive a clearance from the World Health Organisation Indians who have taken Covaxin miss out on travelling to countries like the UK USA among others. As a decision is awaited on the Emergency Use Listing of Covaxin being manufactured in India a top WHO official has said the process of thoroughly evaluating a vaccine for use and recommending it. Technical Advisory Group seeks additional clarification on Covaxin for Emergency Use Listing. Covaxin Who Approval News WHOs Emergency Use Approval For Covaxin Will Facilitate Our Process to Help Other Countries.
Covaxin has demonstrated 778 per cent effectiveness against symptomatic COVID-19 and 652 per cent protection against the new Delta variant. The Hyderabad-based company had in May this year submitted an application to the. The decision on Covaxins WHO EUL could be expected sooner than later with the global health bodys Strategic Advisory Group of. While the WHO has so far approved Covid-19 vaccines of Pfizer-BioNTech AstraZeneca-SK BioSerum Institute of India Johnson Johnson-Janssen Moderna and Sinopharm.
The latest Status of COVID-19 vaccines within. Bharat Biotechs Covaxin and AstraZeneca and Oxford Universitys Covishield are the two widely used vaccines in India. WHO panels four-day meeting begins today. The World Health Organization WHO on Thursday opened up on the delay of approval of the Covaxin in its Emergency Use Listing EUL.
WHO an independent group of experts are scheduled to meet next week to carry out the risk. TRENDING TODAY After 12 hours of. A decision on Covaxins inclusion in the Emergency Use List will be taken in 2-3 months time the Union government informed the Rajya Sabha on Tuesday. The process for approval of a vaccine by WHO consists of four steps.
Supreme Court prefers to wait and watch for Covaxins WHO approval Legal Correspondent NEW DELHI October 29 2021 1627 IST. Foreign Secretary Harsh Vardhan Shringla has said that Bharat Biotechs Covaxin Indias first indigenous Covid-19 vaccine will be approved by the World Health Organisation WHO soon. The status of the assessment for Covaxin is ongoing. WHO and an independent group of experts are scheduled to meet next week to carry out the riskbenefit assessment and arrive at a decision on granting EUL to Covaxin.
Published on August 12 2021 Follow us on Telegram Facebook Twitter Instagram YouTube and Linkedin. Heres a list of countries that have approved the Bharat Biotech vaccine. Bharat Biotech had submitted EOI Expression of Interest on 19 April for its COVID-19 vaccine. The WHO is in the process of evaluating Covaxins clinical trial data for use of EUL.
Top sources told CNN-News18 that representatives of the global health watchdog will meet Union Health Minister Mansukh Mandaviya on Wednesday to hold talks in the matter. The foreign secretary further stressed that its a technical process and not an administrative or a political process. An acceptance of the manufacturers expression of interest EOI a pre-submission meeting between WHO and the manufacturer acceptance of the dossier for review by WHO decision on status. A decision on Bharat Biotechs submission seeking emergency use listing EUL for its Covaxin COVID-19 vaccine will be made in October the World Health Organisation has said.
Hyderabad-based pharmaceutical firm Bharat Biotech which is the company that developed Covaxin has been awaiting the EUL for the COVID-19 shot for months now. A WHO official has said the agencys assessment of made-in-India Covaxin is at an advanced stage and a decision.

Bharat Biotech Hopes To File For Covaxin Approvals By Next June Latest Business News Economic Times Next

Twelve Million Doses To Be Shipped Covaxin Maker Bharat Biotech Has Signed An Agreement With Precisa Medicamentos A Firm In Brazil To In 2021 Agreement Dose Signs

Pin Von Aadithyan Auf Videos011120

259 Likes 1 Comments Ias Academy Mohali Chandigarh Iasmagnus On Instagram Download Magnus Institute App For Exam Study Materials Syllabus Exam




Posting Komentar untuk "Covaxin Who Approval Status Today"